Introduction to Quality Management System (QMS)
QMS When you think of the word quality, what is the first thing that comes to mind? The word quality has more than one definition. Quality could be defined as a feature or characteristic as in the following statement: They are people of integrity, which is a good quality to possess. It can also be thought as to how well something is made, if it meets all required specifications or if there are any apparent defects or non-conformances in the product. Therefore, quality is also defined as a determination of how good or bad something is, or how well it meets customer expectations. One example would be the quality of the paint on a new car. Quality systems focus on the latter definition.
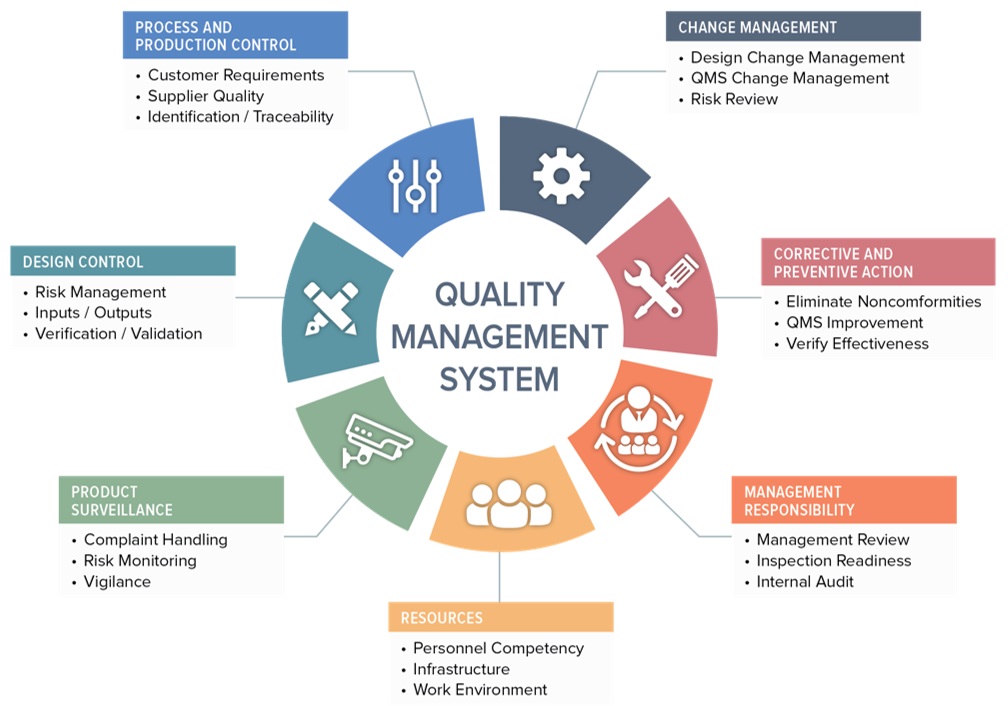
Quality Management Systems (QMS) are intended to help assure that a product or service meets or exceeds the customer’s expectations each and every time. Only by consistently meeting or exceeding the customer’s perception of quality can an organization not merely survive, but grow and thrive.
What is a Quality Management System (QMS)
If we look at QMS in reverse, we can develop a better understanding of its definition. QMS is a System for Managing the Quality of a product or process. Furthermore, QMS is a system for documenting the structure, procedures, responsibilities, and processes needed for effective quality management. The QMS outlines how an organization will produce, document, control, and deliver a product or service possessing customer perceived value.
Why Implement a Quality Management System (QMS)
Multiple benefits result from development and implementation of a robust Quality Management System. Some of the most obvious benefits to implementing QMS are as follows:
- Managing product and process quality enables an organization to consistently meet the needs and wants of their customers through Voice of the Customer (VOC). Increased customer satisfaction results in more sales, increased market share, and a loyal customer base.
- Ensuring that all government regulations and requirements are met with every new product introduction allows marketing products worldwide.
- Reduction of costly rework and/or scrap is realized through implementation and monitoring of process controls.
- Management is able to make decisions based on data, not conjecture. The data collected through the implementation of Statistical Process Control (SPC) and other methods allow management to make decisions based on evidence. Valuable resources are utilized where they will have the most impact on improving process efficiency and reducing quality issues.
- Engagement of the associates in the process and product improvement efforts helps to create a continuous improvement culture within the organization. Through the introduction of Kaizen, 5S, and other quality tools, the associates gradually take mental ownership of the process. Associates invested in the processes they perform are best at identifying opportunities for improvements that will result in better quality, efficiency, and safety.
Developing and implementing a Quality Management System enables organizations of all types to be more efficient and effective. Some have the false impression that the quality system only involves actions performed by personnel within the quality department. The Quality Management System affects multiple processes and departments within an organization from sales, design, development, production, and delivery of the product or service to the customer. The QMS promotes cross-functional communication and interaction throughout the organizational structure, which can result in a more unified and stronger organization.
How to Implement a Quality Management System (QMS)
Implementing a Quality Management System into any organization, either large or small, is not a quick or simple task. It will require an investment in time and resources to successfully implement an effective QMS. Numerous important tasks and processes will require development and implementation. Below is a list of some key areas to consider when implementing a QMS.
Secure Support of Top Management
Support from top management is vital to the success of any QMS implementation. The management team of an organization must be committed to the success of the QMS. Management must be convinced of the positive impact on business efficiency and the bottom line. Also, management should be directly involved in certain elements of the QMS implementation process. Below are a couple of examples:
- Business Analysis: The management team should review their business structure and determine the key areas for implementation of a QMS. The key areas should be determined according to critical to customer requirements. Consider the current needs of the organization and alignment with long-term strategic goals.
- Initial Planning: Management should be actively involved in the planning stages by determining the resources required, assembling the teams, and formulating an implementation plan. In addition, discussions should identify which existing processes will be improved first and confirm strategic or SMART goals.
Increase Awareness
Spread the word; schedule informational meetings across the organization to inform all associates about the pending Quality Management System. Include information regarding how the system will benefit the customers and employees. Explain how QMS works. In addition, include the roles and responsibilities of the associates at each level and in each department.
Provide Expert Training
Proper training is essential for the success of any new product/process or management system introduction. The amount and type of QMS training are determined during the management team’s initial planning phase. The training materials should include a review of the basic concepts and tools used along with information regarding the positive impact that the QMS will provide for the associates and the organization.
Documents and Document Control
Proper documentation is at the heart of a well-functioning Quality Management System. There must be documentation developed to support the implementation, education, deployment, and control functions of the system. Some of the common documents may include:
- Policies
- Procedures
- Quality Manual
- Training Materials
- Work Instructions
- Audit Forms
- Process Maps
- Control Plans
Proper documentation is vital to the success of a QMS and maintaining control of those documents is equally important. A document control system manages the creation, approval, distribution, revision, storage, and disposal of all quality documentation. The system functions to assure everyone is performing tasks, in the same manner, using the correct revision of the document.
Deployment
Deployment of the QMS should follow the implementation plan developed during the planning phase. Document each process and define the current state. Involve the associates in the documentation of the process, utilization of various quality tools, and development of metrics. Once this is complete, the teams can focus on improvement efforts. Select an area or process with the most opportunity for improvement. Quick wins promote “buy-in” by the teams and associates by illustrating the improvements to the process and the benefit to the associates and the company as a whole. There are multiple quality and improvement tools available for use in improving a process. They may include:
- 5S +1
- Kaizen
- Process Flow Charts
- Process Failure Mode and Effects Analysis (PFMEA)
- Value Stream Mapping
The timing of the deployment is generally dependent upon the size of the organizations and the number of facilities that will be included in the implementation of the QMS. Throughout the implementation, a company intranet is an effective tool for communicating progress towards planned objectives and sharing best practices with associates throughout the organization.
Measure and Control
Once deployment has occurred, the various processes within the organization must be controlled and the key process and product characteristics measured and monitored to ensure the continued production of quality products. Manufacturing processes often incorporate SPC methodology. Other methods could include random inspections or routine audits. The specific methods will vary depending on the organization’s size, structure, process, and potential risk.
Audit and Maintain
Routine Product / Process Audits monitor adherence to policies, procedures, and any certification requirements. A schedule should be developed and maintained to assure each department, area, and the process is audited on a regular basis. The timing of the audits will vary dependent upon the organization, process, potential risk, and any regulatory or certification requirements. Audits will reveal whether or not you are actually doing what you said you were going to do to manage quality and ensure conformance to safety / regulatory requirements. Routine audits are effective tools for:
- Identifying inadequacies in your quality management process
- Discovering possible problems that could result in non-compliance penalties
- Maintaining adherence to established processes and quality controls
When an internal auditing process is well planned, implemented, and maintained, the internal audits add considerable value to the quality management process.
Lessons Learned and Continuous Improvement
A very important advantage of implementing a QMS is the ability to use lessons learned from one department or process to make improvements in similar processes across the organization. In addition, for organizations to remain competitive and thrive in today’s world market, they must seek ways to continually improve their processes and product or service quality. Associates at all levels of the organization should be encouraged to look for opportunities for change and improvement every day. Even by implementing small continuous improvements in the processes and work standards, the organization will realize sizable increases in quality, safety, efficiency, and productivity. This ultimately results in a positive impact on the bottom line. Some organizations view continuous improvement as an activity, where others adopt it as a mindset.